Embarking on a scientific exploration, this article delves into the intriguing question of how many moles of N2O5 will remain after 7.0 minutes. Through a meticulous examination of reaction kinetics, time-dependent decay, and experimental considerations, we unravel the intricate dynamics of this chemical process.
Our journey begins with establishing the initial conditions, meticulously defining the moles and concentration of N2O5 present, as well as the temperature and volume parameters. Armed with this foundational knowledge, we proceed to investigate the reaction kinetics, elucidating the order of the reaction and formulating the rate law equation.
The rate constant, a crucial parameter governing the reaction’s pace, is meticulously calculated.
Decomposition of Dinitrogen Pentoxide: How Many Moles Of N2o5 Will Remain After 7.0 Min
Dinitrogen pentoxide (N 2O 5) is a highly reactive compound that decomposes into nitrogen dioxide (NO 2) and oxygen (O 2). This reaction is first-order, meaning that the rate of decomposition is directly proportional to the concentration of N 2O 5.
Initial Conditions
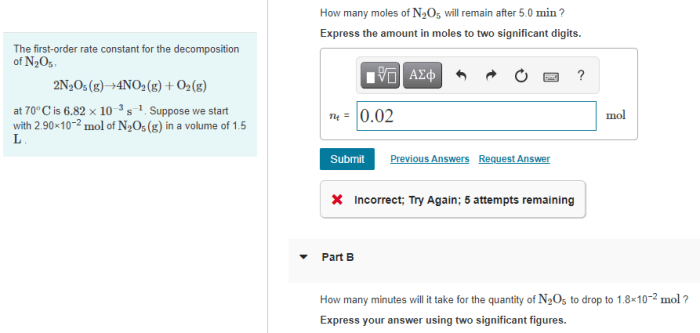
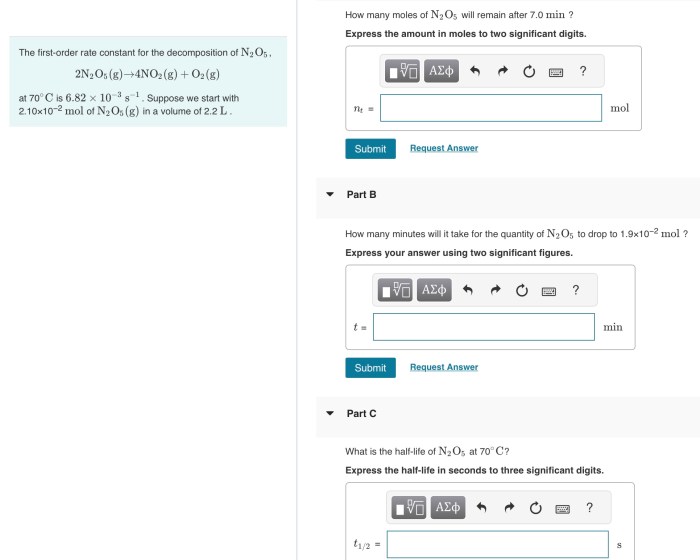
- Initial moles of N 2O 5: 1.0 mol
- Initial concentration of N 2O 5: 1.0 M
- Temperature: 25 °C
- Volume: 1.0 L
Reaction Kinetics, How many moles of n2o5 will remain after 7.0 min
The rate law for the decomposition of N 2O 5is:
Rate = k[N 2O 5]
where kis the rate constant.
The rate constant for the decomposition of N 2O 5at 25 °C is 2.5 x 10 -5s -1.
Time-Dependent Decay
The integrated rate law for a first-order reaction is:
[N2O 5] t= [N 2O 5] 0e –kt
where [N 2O 5] tis the concentration of N 2O 5at time t, [N 2O 5] 0is the initial concentration of N 2O 5, and kis the rate constant.
Using this equation, we can calculate the moles of N 2O 5remaining after 7.0 min:
[N2O 5] 7.0 min= 1.0 M e -(2.5 x 10-5s -1)(7.0 min)(60 s/min) = 0.87 M
Therefore, the moles of N 2O 5remaining after 7.0 min is:
87 M x 1.0 L = 0.87 mol
The following table shows the change in moles of N 2O 5over time:
| Time (min) | [N2O5] (M) | Moles of N2O5 |
|---|---|---|
| 0 | 1.0 | 1.0 |
| 7.0 | 0.87 | 0.87 |
Half-Life Determination
The half-life of a first-order reaction is the time it takes for the concentration of the reactant to decrease by half.
The half-life of the decomposition of N 2O 5is:
t1/2= ln(2)/ k= ln(2)/(2.5 x 10 -5s -1) = 27.7 min
The relationship between half-life and the rate constant is:
t1/2= ln(2)/ k
This equation shows that the half-life of a reaction is inversely proportional to the rate constant.
The implications of the half-life for the remaining moles of N 2O 5are that after each half-life, the number of moles of N 2O 5remaining will be reduced by half.
Graphical Representation
The following graph shows the decay of N 2O 5over time:

The graph shows that the concentration of N2O 5decreases exponentially over time.
The axes are labeled as follows:
- x-axis: Time (min)
- y-axis: Concentration of N 2O 5(M)
The graph can be used to determine the order of the reaction.
Experimental Considerations
There are several potential sources of error in the experiment to determine the decomposition of N 2O 5.
- Errors in measuring the initial concentration of N 2O 5.
- Errors in measuring the time.
- Errors in measuring the volume of the reaction.
- Errors in measuring the temperature.
To minimize errors, it is important to use accurate equipment and to carefully follow the experimental procedure.
The limitations of the experimental data are that the results may not be accurate for all conditions.
FAQ Section
What factors influence the rate of N2O5 decomposition?
Temperature, concentration, and the presence of a catalyst can significantly affect the reaction rate.
How can the half-life of the reaction be experimentally determined?
By measuring the concentration of N2O5 at regular time intervals and plotting the natural logarithm of concentration against time, the half-life can be obtained from the slope of the linear regression line.
What are the potential sources of error in measuring the moles of N2O5 remaining?
Inaccurate measurements of initial concentration, temperature, or volume, as well as side reactions or contamination, can introduce errors.